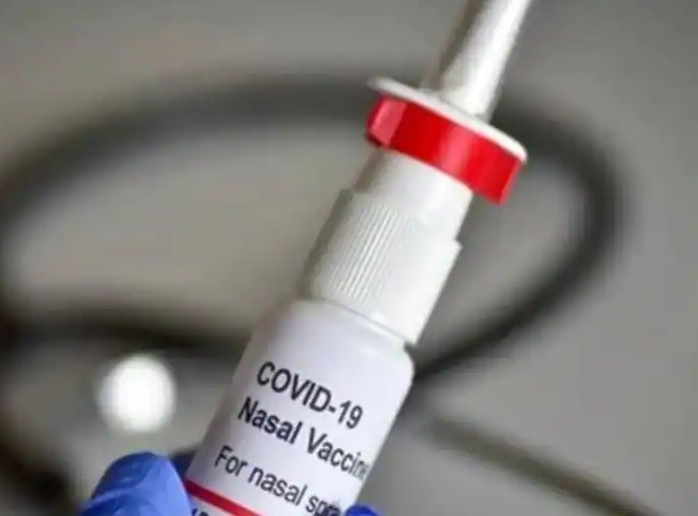
New Delhi: Bharat Biotech’s nasal vaccine was approved in India last week. On Tuesday, the company also gave information about its price.
However, the nasal vaccine will not be administered to those who have taken precautionary or booster doses said head of the country’s vaccine task force Dr N K Arora.
He said, “It (nasal vaccine) is to be administered as a booster first. For example, if a person has already received the precautionary dose then it is not for that person. This is for those who have not taken the precautionary dose yet.”
Dr Arora is chairman of NTAGI’s Covid Working Group, an acronym for the National Technical Advisory Group on Immunisation. The body works on introducing new vaccines and strengthening the universal immunisation programme.
Dr Arora said CoWIN will not accept the fourth dose as part of the vaccine programme. “Let’s say you want to take another fourth dose. There is a concept called ‘antigen sync’. If a person is repeatedly given immunity to a particular type of antigen, the body stops responding, or reacts poorly.”
That is why mRNA vaccines are initially given at an interval of six months, the Vaccine Task Force explained. Later people are taking it at an interval of three months, but in that case it has not helped much. So there is no value in taking the fourth dose at the moment. He said that the nasal vaccine provides a very interesting method of vaccination.